cpy_131_repro_calc
Type: Sample show more
ObjectId: 29160
Created: 8/8/2025 9:51:43 AM by A09) Leppin Christian [christian.leppin@rub.de]
Updated: 8/13/2025 5:47:18 PM by A09) Leppin Christian [christian.leppin@rub.de]
Access: Public Sort Code (asc): 0
Description: The nanoparticles were provided from the Schulz-Group. Synthesis description: For the synthesis of spherical Co3O4 nanoparticles, 1.164 g Co(NO3)2·6 H2O (4.0 mmol) was dissolved in 200 mL water in an open reactor vessel. Subsequently, 3.24 mL of ammonium hydroxide solution and 240 µL of H2O2 were added. The black reaction mixture was then heated to 60 °C for 15 minutes. A solution containing 0.58 g Co(NO3)2·6 H2O (2 mmol) dissolved in 80 mL water was added and the reaction temperature was maintained at 60 °C for 3 hours. After cooling to room temperature, the nanoparticles were collected by centrifugation at 3000 rpm, washed with water and acetone and dried in air. The particles were then heated at a rate of 4.5 °C min-1 to 300 °C, where the temperature was maintained for 15 minutes before cooling to room temperature.
[no file attached]
: Co-O
Associated Objects
-
PXRD_cpy_131_repro_calc_result
Raw DocumentResults from fitting Pseudo-Voigt profiles to the pattern and applying Scherrer equation.
PXRD_cpy_131_repro_calc_result.txt
-
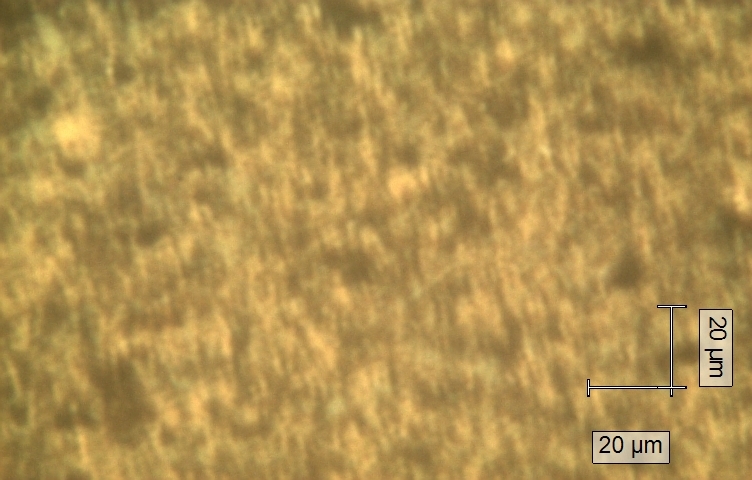
TEM_Image_CPY131_repro_calc
TEM imageTEM image kindly provided by Georg Bendt. The transmission electron micrograph (TEM) was recorded using a JEOL 2200FS transmission electron microscope operated at 200 kV.
TEM_Image_CPY131_repro_calc.png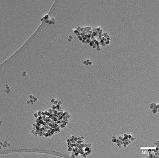
-
PXRD_cpy_131_repro_calc_result
XRD ImageResult plot from fitting Pseudo-Voigt profiles to the XRD pattern and applying Scherrer equation.
PXRD_cpy_131_repro_calc_result.tif
-
CL20250803_AnalyzePXRD
Python Script (py, pkl, zip, ipynb)Python notebook to carry out the analysis of the PXRD pattern and calculate the pattern for bulk Co3O4 from CIF file (1526734)[1]. [1] Kotousova, I. S.; Polyakov, S. M. Electron diffraction study of Co3O4. Kristallografiya 1972, 17 (3), 661.
CL20250803_AnalyzePXRD.ipynb -
CL20250807_AnalyzeSEM
Python Script (py, pkl, zip, ipynb) CL20250807_AnalyzeSEM.ipynb
-
PXRD_cpy_131_repro_calc
XRD Integ. Raw ZIP (xy)PXRD of Co3O4 kindly provided by Dr. Georg Bendt. The PXRD patterns were recorded on a Bruker D8 Advance powder diffractometer using Bragg-Brentano geometry at room temperature with Cu Kα radiation (λ = 1.5418 Å, 40 kV, and 40 mA). The powder samples were examined in the range between 5 ° and 90 ° (2θ) with a step size of 0.01 ° (2θ).
PXRD_cpy_131_repro_calc.xy
Referenced Objects (Reverse Association)
-
ChemCatChem, 2025 (Leppin)
PublicationLeppin C., Placke-Yan C., Bendt G., Hernandez S., Tschulik K., Schulz S., Linnemann J.: Interfacial Softening and Electrolyte Uptake in Co₃O₄ OER Catalysts: Insight from Operando Spectroscopy and Fast EQCM-D. ChemCatChem, 2025 (accepted), DOI: 10.1002/cctc.202501104
-
#10_Co3O4_Nanoparticle_Electrode
SampleElabID: CL250528_001_Co3O4_QCM#10, Preparation Procedure: The Co3O4 nanoparticle electrodes with a small catalyst loading were fabricated by spin-casting (Laurell WS-400BX-6NPP/LITE) 20 µL of Co3O4 ink at 4000 rpm for 5 min onto a gold-coated quartz crystal microbalance (QCM, BeamTech) resonator, which served as the current collector. The ink was prepared by dispersing Co3O4 nanoparticles (cpy_131_repro_calc) in Milli-Q water to achieve a concentration of 0.1 mg mL⁻¹. The exact catalyst loading cannot be directly derived from the dispersion’s concentration and cast volume. It is, however, approximated to be 1.7 µg cm⁻² at the maximum, as excess solution is expelled from the substrate during spinning.
-
#11_Co3O4_Nanoparticle_Electrode
SampleElab Identifier: CL250528_001_Co3O4_QCM#11, Preparation Procedure: The Co3O4 nanoparticle electrodes with a small catalyst loading were fabricated by spin-casting (Laurell WS-400BX-6NPP/LITE) 20 µL of Co3O4 ink at 4000 rpm for 5 min onto a gold-coated quartz crystal microbalance (QCM, BeamTech) resonator, which served as the current collector. The ink was prepared by dispersing Co3O4 nanoparticles (cpy_131_repro_calc) in Milli-Q water to achieve a concentration of 0.1 mg mL⁻¹. The exact catalyst loading cannot be directly derived from the dispersion’s concentration and cast volume. It is, however, approximated to be 1.7 µg cm⁻² at the maximum, as excess solution is expelled from the substrate during spinning.
-
#43_Co3O4_Nanoparticle_Electrode
SampleElabID: CL20250726_001_Co3O4#43, Preparation Procedure: The Co3O4 nanoparticle electrodes with a high catalyst loading (42.7 µg cm-2) were fabricated by drop-casting 100 µL of Co3O4 ink onto a gold-coated quartz crystal microbalance (QCM) resonator, which served as the substrate. The ink was prepared by dispersing Co3O4 nanoparticles in Milli-Q water to achieve a concentration of 0.5 mg mL⁻¹.
-
#48_Co3O4
SampleElab Identifier: , Preparation Procedure: The Co3O4 nanoparticle electrodes with a high catalyst loading (42.7 µg cm-2) were fabricated by drop-casting 100 µL of Co3O4 ink onto a gold-coated quartz crystal microbalance (QCM) resonator, which served as the substrate. The ink was prepared by dispersing Co3O4 nanoparticles in Milli-Q water to achieve a concentration of 0.5 mg mL⁻¹.
-
#21_Co3O4_Nanoparticle_Electrode
SampleELabID: ,Preparation Procedure: The Co3O4 nanoparticle electrodes with a small catalyst loading were fabricated by spin-casting (Laurell WS-400BX-6NPP/LITE) 20 µL of Co3O4 ink at 4000 rpm for 5 min onto a gold-coated quartz crystal microbalance (QCM, BeamTech) resonator, which served as the current collector. The ink was prepared by dispersing Co3O4 nanoparticles (cpy_131_repro_calc) in Milli-Q water to achieve a concentration of 0.1 mg mL⁻¹. The exact catalyst loading cannot be directly derived from the dispersion’s concentration and cast volume. It is, however, approximated to be 1.7 µg cm⁻² at the maximum, as excess solution is expelled from the substrate during spinning.
-
CL20250721_011_Co3O4_#21_Image4_Spot2_30mW_1s_small_loading
Raw Document CL20250721_011_Co3O4_#21_Image4_Spot2_30mW_1s_small_loading.wdf -
CL20250721_016_Co3O4_#21_Image2_Spot1_30mW_1s_small_loading
Raw Document CL20250721_016_Co3O4_#21_Image2_Spot1_30mW_1s_small_loading.wdf -
CL20250727_001_Co3O4#43_0p1MKOHpristine_high_loading
Raw Document CL20250727_001_Co3O4#43_0p1MKOHpristine_high_loading.wdf